Define carbon dating 14
Contents:
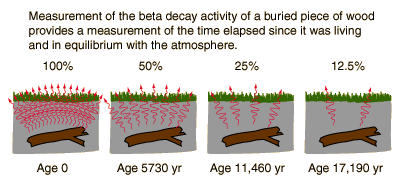
This CO 2 is used in photosynthesis by plants, and from here is passed through the food chain see figure 1, below. Every plant and animal in this chain including us! When living things die, tissue is no longer being replaced and the radioactive decay of 14 C becomes apparent. Around 55, years later, so much 14 C has decayed that what remains can no longer be measured. In 5, years half of the 14 C in a sample will decay see figure 1, below.
Therefore, if we know the 14 C: Unfortunately, neither are straightforward to determine. The amount of 14 C in the atmosphere, and therefore in plants and animals, has not always been constant. For instance, the amount varies according to how many cosmic rays reach Earth. Luckily, we can measure these fluctuations in samples that are dated by other methods. Tree rings can be counted and their radiocarbon content measured. A huge amount of work is currently underway to extend and improve the calibration curve.
In we could only calibrate radiocarbon dates until 26, years. Now the curve extends tentatively to 50, years.
Radiocarbon dates are presented in two ways because of this complication. The uncalibrated date is given with the unit BP radiocarbon years before The calibrated date is also presented, either in BC or AD or with the unit calBP calibrated before present - before The second difficulty arises from the extremely low abundance of 14 C. Many labs now use an Accelerator Mass Spectrometer AMS , a machine that can detect and measure the presence of different isotopes, to count the individual 14 C atoms in a sample.
Dating history
Australia has two machines dedicated to radiocarbon analysis, and they are out of reach for much of the developing world. In addition, samples need to be thoroughly cleaned to remove carbon contamination from glues and soil before dating. This is particularly important for very old samples.
Because of this, radiocarbon chemists are continually developing new methods to more effectively clean materials. These new techniques can have a dramatic effect on chronologies. With the development of a new method of cleaning charcoal called ABOx-SC , Michael Bird helped to push back the date of arrival of the first humans in Australia by more than 10, years.
Moving away from techniques, the most exciting thing about radiocarbon is what it reveals about our past and the world we live in. Radiocarbon dating was the first method that allowed archaeologists to place what they found in chronological order without the need for written records or coins. In the 19th and early 20th century incredibly patient and careful archaeologists would link pottery and stone tools in different geographical areas by similarities in shape and patterning.
Then, by using the idea that the styles of objects evolve, becoming increasing elaborate over time, they could place them in order relative to each other - a technique called seriation. In this way large domed tombs known as tholos or beehive tombs in Greece were thought to predate similar structures in the Scottish Island of Maeshowe. This supported the idea that the classical worlds of Greece and Rome were at the centre of all innovations.
Some of the first radiocarbon dates produced showed that the Scottish tombs were thousands of years older than those in Greece. The barbarians of the north were capable of designing complex structures similar to those in the classical world. Other high profile projects include the dating of the Turin Shroud to the medieval period, the dating of the Dead Sea Scrolls to around the time of Christ, and the somewhat controversial dating of the spectacular rock art at Chauvet Cave to c.
- Navigation menu!
- How Does Radiocarbon-14 Dating Work?.
- Explainer: what is radiocarbon dating and how does it work?;
- carbon dating!
- chat rooms for dating.
Radiocarbon dating has also been used to date the extinction of the woolly mammoth and contributed to the debate over whether modern humans and Neanderthals met. Samples for dating need to be converted into a form suitable for measuring the 14 C content; this can mean conversion to gaseous, liquid, or solid form, depending on the measurement technique to be used.
Before this can be done, the sample must be treated to remove any contamination and any unwanted constituents. Particularly for older samples, it may be useful to enrich the amount of 14 C in the sample before testing. This can be done with a thermal diffusion column. Once contamination has been removed, samples must be converted to a form suitable for the measuring technology to be used. For accelerator mass spectrometry , solid graphite targets are the most common, although gaseous CO 2 can also be used.
The quantity of material needed for testing depends on the sample type and the technology being used. There are two types of testing technology: For beta counters, a sample weighing at least 10 grams 0. For decades after Libby performed the first radiocarbon dating experiments, the only way to measure the 14 C in a sample was to detect the radioactive decay of individual carbon atoms.
Libby's first detector was a Geiger counter of his own design. He converted the carbon in his sample to lamp black soot and coated the inner surface of a cylinder with it. This cylinder was inserted into the counter in such a way that the counting wire was inside the sample cylinder, in order that there should be no material between the sample and the wire. Libby's method was soon superseded by gas proportional counters , which were less affected by bomb carbon the additional 14 C created by nuclear weapons testing.
These counters record bursts of ionization caused by the beta particles emitted by the decaying 14 C atoms; the bursts are proportional to the energy of the particle, so other sources of ionization, such as background radiation, can be identified and ignored. The counters are surrounded by lead or steel shielding, to eliminate background radiation and to reduce the incidence of cosmic rays. In addition, anticoincidence detectors are used; these record events outside the counter, and any event recorded simultaneously both inside and outside the counter is regarded as an extraneous event and ignored.
The other common technology used for measuring 14 C activity is liquid scintillation counting, which was invented in , but which had to wait until the early s, when efficient methods of benzene synthesis were developed, to become competitive with gas counting; after liquid counters became the more common technology choice for newly constructed dating laboratories.
How Does Carbon Dating Work
The counters work by detecting flashes of light caused by the beta particles emitted by 14 C as they interact with a fluorescing agent added to the benzene. Like gas counters, liquid scintillation counters require shielding and anticoincidence counters. For both the gas proportional counter and liquid scintillation counter, what is measured is the number of beta particles detected in a given time period.
This provides a value for the background radiation, which must be subtracted from the measured activity of the sample being dated to get the activity attributable solely to that sample's 14 C. In addition, a sample with a standard activity is measured, to provide a baseline for comparison.
The ions are accelerated and passed through a stripper, which removes several electrons so that the ions emerge with a positive charge.
A particle detector then records the number of ions detected in the 14 C stream, but since the volume of 12 C and 13 C , needed for calibration is too great for individual ion detection, counts are determined by measuring the electric current created in a Faraday cup. Any 14 C signal from the machine background blank is likely to be caused either by beams of ions that have not followed the expected path inside the detector, or by carbon hydrides such as 12 CH 2 or 13 CH. A 14 C signal from the process blank measures the amount of contamination introduced during the preparation of the sample.
These measurements are used in the subsequent calculation of the age of the sample. The calculations to be performed on the measurements taken depend on the technology used, since beta counters measure the sample's radioactivity whereas AMS determines the ratio of the three different carbon isotopes in the sample. To determine the age of a sample whose activity has been measured by beta counting, the ratio of its activity to the activity of the standard must be found.
To determine this, a blank sample of old, or dead, carbon is measured, and a sample of known activity is measured.
carbon dating
The additional samples allow errors such as background radiation and systematic errors in the laboratory setup to be detected and corrected for. The results from AMS testing are in the form of ratios of 12 C , 13 C , and 14 C , which are used to calculate Fm, the "fraction modern". Both beta counting and AMS results have to be corrected for fractionation. The calculation uses 8,, the mean-life derived from Libby's half-life of 5, years, not 8,, the mean-life derived from the more accurate modern value of 5, years.
The reliability of the results can be improved by lengthening the testing time. Radiocarbon dating is generally limited to dating samples no more than 50, years old, as samples older than that have insufficient 14 C to be measurable. Older dates have been obtained by using special sample preparation techniques, large samples, and very long measurement times.
These techniques can allow measurement of dates up to 60, and in some cases up to 75, years before the present.
- Radiocarbon dating.
- jewish girl dating asian guy.
- married after 2 months of dating.
This was demonstrated in by an experiment run by the British Museum radiocarbon laboratory, in which weekly measurements were taken on the same sample for six months. The measurements included one with a range from about to about years ago, and another with a range from about to about Errors in procedure can also lead to errors in the results. The calculations given above produce dates in radiocarbon years: To produce a curve that can be used to relate calendar years to radiocarbon years, a sequence of securely dated samples is needed which can be tested to determine their radiocarbon age.
The study of tree rings led to the first such sequence: These factors affect all trees in an area, so examining tree-ring sequences from old wood allows the identification of overlapping sequences. In this way, an uninterrupted sequence of tree rings can be extended far into the past.
Carbon Dating
The first such published sequence, based on bristlecone pine tree rings, was created by Wesley Ferguson. Suess said he drew the line showing the wiggles by "cosmic schwung ", by which he meant that the variations were caused by extraterrestrial forces. It was unclear for some time whether the wiggles were real or not, but they are now well-established.
A calibration curve is used by taking the radiocarbon date reported by a laboratory, and reading across from that date on the vertical axis of the graph. The point where this horizontal line intersects the curve will give the calendar age of the sample on the horizontal axis. This is the reverse of the way the curve is constructed: Over the next thirty years many calibration curves were published using a variety of methods and statistical approaches.
The improvements to these curves are based on new data gathered from tree rings, varves , coral , plant macrofossils , speleothems , and foraminifera.
Stone and metal cannot be dated but pottery may be dated through surviving residue such as food particles or paint that uses organic material 8. Several formats for citing radiocarbon results have been used since the first samples were dated. This is particularly important for very old samples. Dates on organic material recovered from strata of interest can be used to correlate strata in different locations that appear to be similar on geological grounds. Australia has two machines dedicated to radiocarbon analysis, and they are out of reach for much of the developing world. When the animal or plant dies, it stops exchanging carbon with its environment, and from that point onwards the amount of 14 C it contains begins to decrease as the 14 C undergoes radioactive decay.
The INTCAL13 data includes separate curves for the northern and southern hemispheres, as they differ systematically because of the hemisphere effect. The southern curve SHCAL13 is based on independent data where possible, and derived from the northern curve by adding the average offset for the southern hemisphere where no direct data was available.