Evolution dating techniques
Contents:
Is Dating Really Important? Index For This Page. I wish this page was unnecessary. Because of the distortions and lies spread by fundamentalists about scientific dating there is a need for a centralized source of information on the topic. A few examples of such lies are presented at the very bottom of this page. For each dating or chronological method there is a link in the box at right to take you to that section of this page.
The thickness of the hydration rind can be identified in petrographic thin sections cut normal to the surface and observed under a microscope. A Chemistry World subscription brings you all the research, news and views from the global chemical science community. Samples are exposed to neutrons in a nuclear reactor. See more information about "Strata" Smith and his original geologic map of England. Only registered users can comment on this article. Friedman and Smith reasoned that the degree of hydration observed on an obsidian artifact could tell archaeologists how long it had been since that surface was created by a flintknapper. Carbon, though, is continuously created through collisions of neutrons generated by cosmic rays with nitrogen in the upper atmosphere and thus remains at a near-constant level on Earth.
There, you will find a brief description of the method, plus links to take you to other webpages with more extensive information. Dating is not necessary to demonstrate that evolution is a fact. Chronological sequence is all that is really required. However, human beings love to see factual precision, and we want to know how old something is.
Please remember that all dating methods, even those termed "absolute," are subject to margins of error. We say the Earth is 4.
Search form
That is a very small amount of possible error range. There are 20 methods shown here. Modern studies almost always use two or more methods to confirm dating work and to build confidence in the results obtained. Overview of Scientific Dating Methods.
Navigation menu
This is an excellent overview of dating methodologies, and is a chapter in a textbook on Archaeology. You may find it useful for the clear definitions, and for excellent links on a variety of topic. Many of these links also appear where appropriate below. Back to Page Index.
Steno's Law - The Law of Superposition: A bit of history about Nicolas Steno, who formulated the Law of Superposition. James Hutton and William Smith advanced the concept of geologic time and strengthened the belief in an ancient world. Hutton, a Scottish geologist, first proposed formally the fundamental principle used to classify rocks according to their relative ages.
- How reliable is geologic dating?.
- free dating chat singles.
- Your browser is not supported;
- dress dating games.
- Create your free account.
- indian dating affiliate.
- .
He concluded, after studying rocks at many outcrops, that each layer represented a specific interval of geologic time. Further, he proposed that wherever uncontorted layers were exposed, the bottom layer was deposited first and was, therefore, the oldest layer exposed; each succeeding layer, up to the topmost one, was progressively younger.
The Major Divisions of Geologic Time are shown here, arranged in chronological order with the oldest division at the bottom, the youngest at the top. Relative Time, Superposition and Cross-cutting Relationships: Geologic intrusions, faults and unconformities are explained and pictured. Stratigraphy is the study of strata, or layers. Specifically, stratigraphy refers to the application of the Law of Superposition to soil and geological strata containing archaeological materials in order to determine the relative ages of layers.
Radiometric dating
Cross-dating is a technique used to take advantage of consistencies in stratigraphy between parts of a site or different sites, and objects or strata with a known relative chronology. A specialized form of cross-dating, using animal and plant fossils, is known as biostratigraphy. Correlation means matching the order of geologic events in one place with the order of geologic events in another place.
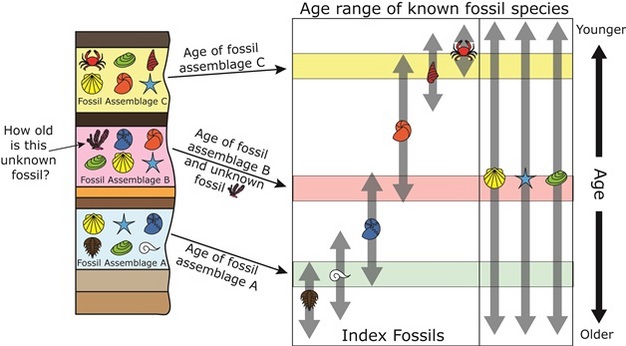
By far, the most widespread method of correlation uses fossils Geologic Time: Keyed to the relative time scale are examples of index fossils, the forms of life which existed during limited periods of geologic time and thus are used as guides to the age of the rocks in which they are preserved. William "Strata" Smith, a civil engineer and surveyor, was well acquainted with areas in southern England where "limestone and shales are layered like slices of bread and butter. Using these key or index fossils as markers, Smith could identify a particular layer of rock wherever it was exposed.
Because fossils actually record the slow but progressive development of life, scientists use them to identify rocks of the same age throughout the world. See more information about "Strata" Smith and his original geologic map of England. Click on the map to see a larger version. The Principles of Dendochronology. Dendrochronology -- Tree Rings: Tree-Ring dating is based on the principle that the growth rings on certain species of trees reflect variations in seasonal and annual rainfall.
Trees from the same species, growing in the same area or environment will be exposed to the same conditions, and hence their growth rings will match at the point where their lifecycles overlap. Earth's oldest living inhabitant "Methuselah" at 4, years, has lived more than a millennium longer than any other tree.
See Oldest Living Organism. The Sheffield Laboratory now has a continuous master sequence for England going back to about BC.
Accuracy of Fossils and Dating Methods
This is made up of numerous regional tree-ring chronologies, particularly in the medieval and post-medieval periods, for which the laboratory now has more than reference chronologies from many areas. The Ultimate Tree-ring Pages: This really must be the ultimate web resource for this topic. You will find information about tree-rings, current research, and examples of practical applications of this science. Potassium-argon dating , Argon-argon dating , Carbon or Radiocarbon , and Uranium series.
All of these methods measure the amount of radioactive decay of chemical elements; the decay occurs in a consistent manner, like a clock, over long periods of time. Thermo-luminescence , Optically stimulated luminescence , and Electron spin resonance. All of these methods measure the amount of electrons that get absorbed and trapped inside a rock or tooth over time. A particular isotope of a particular element is called a nuclide. Some nuclides are inherently unstable.
That is, at some point in time, an atom of such a nuclide will undergo radioactive decay and spontaneously transform into a different nuclide. This transformation may be accomplished in a number of different ways, including alpha decay emission of alpha particles and beta decay electron emission, positron emission, or electron capture. Another possibility is spontaneous fission into two or more nuclides.
While the moment in time at which a particular nucleus decays is unpredictable, a collection of atoms of a radioactive nuclide decays exponentially at a rate described by a parameter known as the half-life , usually given in units of years when discussing dating techniques.
After one half-life has elapsed, one half of the atoms of the nuclide in question will have decayed into a "daughter" nuclide or decay product. In many cases, the daughter nuclide itself is radioactive, resulting in a decay chain , eventually ending with the formation of a stable nonradioactive daughter nuclide; each step in such a chain is characterized by a distinct half-life. In these cases, usually the half-life of interest in radiometric dating is the longest one in the chain, which is the rate-limiting factor in the ultimate transformation of the radioactive nuclide into its stable daughter.
Isotopic systems that have been exploited for radiometric dating have half-lives ranging from only about 10 years e. For most radioactive nuclides, the half-life depends solely on nuclear properties and is essentially a constant. It is not affected by external factors such as temperature , pressure , chemical environment, or presence of a magnetic or electric field. For all other nuclides, the proportion of the original nuclide to its decay products changes in a predictable way as the original nuclide decays over time.
This predictability allows the relative abundances of related nuclides to be used as a clock to measure the time from the incorporation of the original nuclides into a material to the present.
The basic equation of radiometric dating requires that neither the parent nuclide nor the daughter product can enter or leave the material after its formation. The possible confounding effects of contamination of parent and daughter isotopes have to be considered, as do the effects of any loss or gain of such isotopes since the sample was created. It is therefore essential to have as much information as possible about the material being dated and to check for possible signs of alteration. Alternatively, if several different minerals can be dated from the same sample and are assumed to be formed by the same event and were in equilibrium with the reservoir when they formed, they should form an isochron.
This can reduce the problem of contamination. In uranium—lead dating , the concordia diagram is used which also decreases the problem of nuclide loss. Finally, correlation between different isotopic dating methods may be required to confirm the age of a sample.
Evolution: Library: Radiometric Dating
For example, the age of the Amitsoq gneisses from western Greenland was determined to be 3. Accurate radiometric dating generally requires that the parent has a long enough half-life that it will be present in significant amounts at the time of measurement except as described below under "Dating with short-lived extinct radionuclides" , the half-life of the parent is accurately known, and enough of the daughter product is produced to be accurately measured and distinguished from the initial amount of the daughter present in the material.
The procedures used to isolate and analyze the parent and daughter nuclides must be precise and accurate. This normally involves isotope-ratio mass spectrometry. The precision of a dating method depends in part on the half-life of the radioactive isotope involved. For instance, carbon has a half-life of 5, years. After an organism has been dead for 60, years, so little carbon is left that accurate dating cannot be established. On the other hand, the concentration of carbon falls off so steeply that the age of relatively young remains can be determined precisely to within a few decades.
If a material that selectively rejects the daughter nuclide is heated, any daughter nuclides that have been accumulated over time will be lost through diffusion , setting the isotopic "clock" to zero. The temperature at which this happens is known as the closure temperature or blocking temperature and is specific to a particular material and isotopic system.
These temperatures are experimentally determined in the lab by artificially resetting sample minerals using a high-temperature furnace. As the mineral cools, the crystal structure begins to form and diffusion of isotopes is less easy. At a certain temperature, the crystal structure has formed sufficiently to prevent diffusion of isotopes. This temperature is what is known as closure temperature and represents the temperature below which the mineral is a closed system to isotopes.
Thus an igneous or metamorphic rock or melt, which is slowly cooling, does not begin to exhibit measurable radioactive decay until it cools below the closure temperature.